Standards for Sodium Carboxymethylcellulose/ Polyanionic cellulose
How to dissolve CMC
The method of dissolving CMC and the extent of agitation (shear) during dissolution, will ultimately influence the final viscosity of the solution. The solvent, the cchemical composition of the CMC and the shear history of the final solution affect the dissolution properties of CMC, i.e. hydration of the CMC molecules. This means that standardized conditions for CMC dissolution are essential for viscosity control of the resulting solution. The principle of dissolving CMC is to wet all particles as quickly as possible before the viscosity starts to develop. CMC is by nature hydrophilic (“water-loving”), which means that the CMC particles will instantly start to swell (hydrate) and dissolve when dispersed in water. Therefore, the mixing device used must be efficient enough to keep the entire liquid in motion to avoid agglomeration or lump formation. The mixer should create a strong downstream flow in the center of the dissolution tank and CMC should be added to the vortex formed by the stirrer (Figure 1). It is essential to emphasize that the rate of CMC addition must be slow enough (and even) to permit the particles to become individually wetted.
Addition can be done through a funnel or more preferably with an inductor. however, the rate of addition must be balanced to minimize viscosity build up of the aqueous phase while the CMC is being added.
CMC shows good solubility in both cold and hot water. The rate of dissolution increases at elevated temperatures because the viscosity of the solvent and of the developing CMC solution is lower at high temperature. The dissolution rate of the CMC molecule is independent of temperature. When heating is possible, a suitable (and recommended) temperature to prepare a CMC solution is at about 50-60 °C. The dissolution rate of CMC also depends on the particle size. Provided that all particles have been individually wetted and no lumping occurs, a fine powder will always dissolve faster compared to a more coarse material.
Production Chart
CMC is produced from cellulose and monochloroacetic acid (MCA) and with sodium hydroxide (NaOH) as the third essential ingredient. The different steps in the CMC
process are described in the flow sheet below.
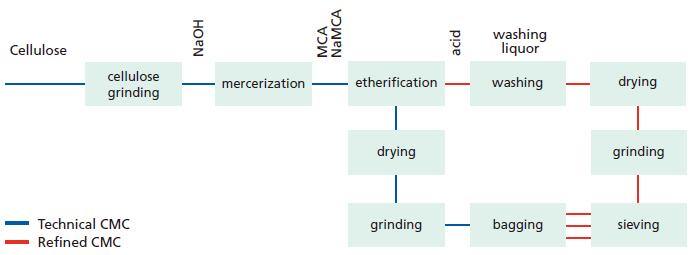
The cellulose is carefully selected to meet the very strict quality requirements of the end product. It is first treated with sodium hydroxide. Alkali cellulose is formed in the reaction between cellulose and sodium hydroxide. This is a crucial step in the process to
ensure that the cellulose is homogeneously converted to alkali cellulose.
The alkali treatment step is commonly known as mercerization. The alkali cellulose is accessible and reactive towards monochloroacetic acid, which is added to the reactor either as free acid—MCA, or as its sodium salt—NaMCA.
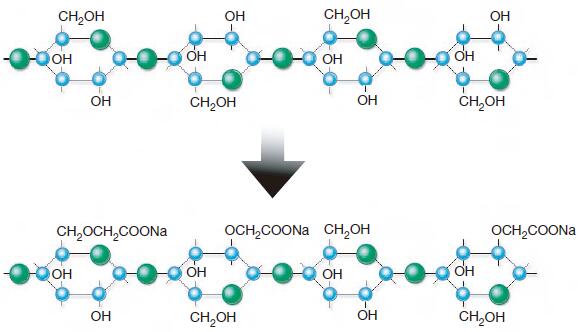
After completion of the different reaction steps the product contains about 25-35% of by-product salts (sodium chloride and sodium glycolate). It can be dried as it is (technical grade CMC) or neutralized and refined by washing either to minimum 98% purity (Technical purified grades) or minimum 99.5% purity (Food, pharma or cosmetic grades).
The basic elements of PAC (polyanionic cellulose) are similar to those of CMC. It is, however, made from specially selected cellulose raw material and manufactured under specific process conditions that ensure the unique characteristics typical of PAC.
Industry Application

